COVID-19 | The Health Ministry appears in the dark about the details of a reported donation of 500,000 doses of a Covid-19 vaccine candidate from the United Arab Emirates to be trialled in Malaysia.
Asked about the donation today, Health director-general Dr Noor Hisham Abdullah said he had “heard” about it but called it “hearsay”.
This is because no documents about the vaccine candidate had been submitted to the National Pharmaceutical Regulatory Agency (NPRA) for review yet.
“We heard about the donation (where) about 500,000 vaccines will be donated to our country.
“But more importantly, it has to go through the NPRA registration (process). Once we get the registration, then only we will allow the vaccine to be used in this country,” Hisham answered during a press conference in Putrajaya this evening.
Government newswire Bernama previously reported that the UAE government intends to donate half-a-million vaccines doses for third-phase clinical trials in Malaysia.

The Yang di-Pertuan Agong Sultan Abdullah Sultan Ahmad Shah is presently on a special visit in the UAE at the invitation of Abu Dhabi Crown Prince Mohamed Zayed Al Nahyan, reportedly to discuss the donation.
Elaborating, Hisham supposed that the donated vaccines were “most probably” from China but could not confirm when asked who the vaccine manufacturer was.
“That is why we are waiting for the whole document [...] now it is hearsay rather than a confirmation,” he said.
“Nothing has been submitted to the NPRA yet at the moment,” the top official replied when asked if the ministry was aware of what vaccine exactly the UAE intended to donate.
Hisham repeatedly stressed that any vaccine will first need to be vetted by the NPRA no matter how it is procured.
“Although the procurement is from UAE with China [...] they will need to appoint a company to facilitate the registration in our country. Once it has been registered, then NPRA will monitor it in terms of the safety and efficacy of the vaccine,” he said.
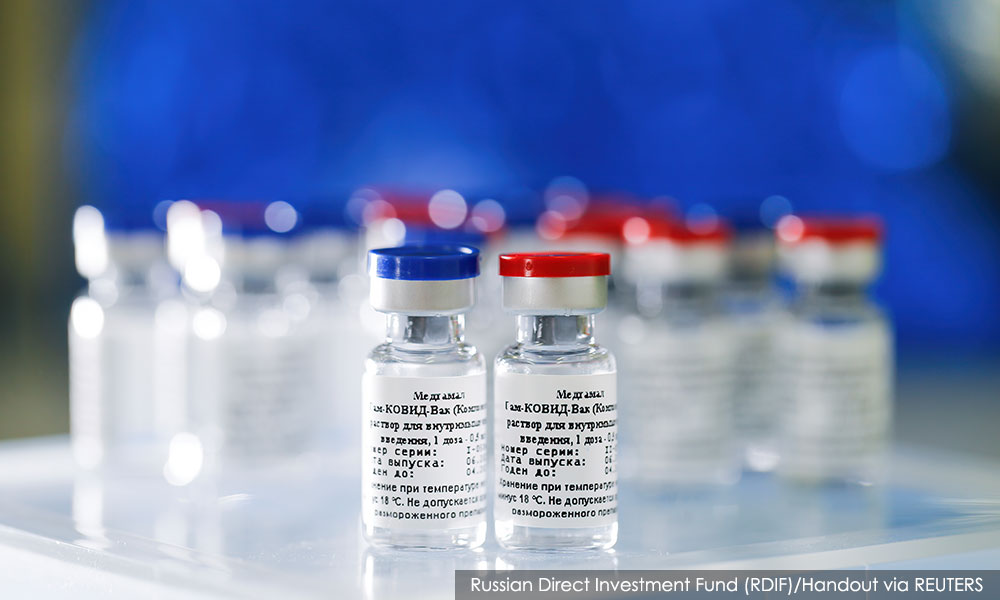
The UAE has not developed any Covid-19 vaccine of its own but is currently hosting efficacy trials for two Covid-19 vaccine candidates.
One candidate is developed by Russia's Gamaleya Research Institute (above), while the other candidate is a collaboration between Sinopharm and the Wuhan Institute of Biological Products.
Malaysia is currently hosting one Covid-19 vaccine trial, which uses an experimental vaccine from the Institute of Medical Biology Chinese Academy of Medical Sciences (IMBCAMS).
The trial is being conducted in eight public hospitals and would involve 3,000 participants.
Ordinarily, the drugs to be used in clinical trials are provided by the trial's sponsor - typically a pharmaceutical company that developed the drug.
Four months to assess Pfizer vaccine
Meanwhile, Hisham confirmed that the ministry received an application from US pharmaceutical company Pfizer on Dec 15 to register its Covid-19 vaccine with the NPRA.
He expects the agency to take up to four months to consider the application.
“We expect that the correspondence with the company will begin at the end of this month (Dec 2020). The NPRA has also identified 11 medical experts in relevant fields to look at the documents submitted to the ministry.
“We might take about 90 to 120 days, but we will prioritise to access the safety and efficacy of the vaccine,” he said.
The Malaysian government previously signed an agreement to procure 12.9 million doses of Pfizer’s vaccine - which will be enough to vaccinate 20 percent of the population.
Malaysia has also signed a deal to obtain vaccines for 10 percent of the population through the Covid-19 Vaccine Global Access (Covax) plan and is slated to sign a deal with the UK firm AstraZeneca today to cover another 20 percent.
China has agreed to give Malaysia priority access to vaccines developed by the country, while Malaysia is still negotiating deals to ensure there would be sufficient Covid-19 vaccines for at least 70 percent of the population.

