UPDATED May 26 | A few changes have been made for the second rollout to people above 18, including an updated advisory for nursing women.
- UPDATED May 22 | This guide was originally written for the first round of AstraZeneca opt-in registrations on May 2. It has since been rewritten for the second round on May 23, as well as adding findings from the latest research on the vaccine.
KINIGUIDE | For the second time, the government is accepting registrations from noon on May 22 for those interested in receiving the AstraZeneca vaccine against Covid-19.
This time, priority will be given to people above 60 years old, and some appointment slots will be set aside for offline registrations. In addition, it is being rolled out in some states outside the Klang Valley.
The vaccine has been controversial for its risk of exceedingly rare but life-threatening blood clots, although its benefits still outweighed the risk posed by Covid-19 itself.
Here is what you should know about the AstraZeneca vaccine and how to get it if you happened to live in the Klang Valley, Penang, Johor, Kuching, or Miri. If you are below 60 years old but you're keen, read on, because you are not left out.
For those who prefer a shorter read, the government has also prepared a two-page fact sheet, available here.
What is happening?
The government has set up a separate channel for those who want the Covid-19 vaccine developed by AstraZeneca and the University of Oxford.
Registration opens for 1,240,000 people at noon tomorrow (May 23) on a first-come-first-served basis. The vaccine appointments themselves will be available from June 7 to July 27.
Residents of the Klang Valley, Penang, Johor and Sarawak are eligible as long as they have already registered for the National Covid-19 Immunisation Programme (NIP). This is due to the high number of Covid-19 cases in these areas.
However, registration is open only for those above 60 years old from May 23 until May 26. If there are any appointment slots still available on May 26, registration will be opened to all residents who are above 18 years old from noon onwards.
Over 228,000 appointments have been taken up by senior citizens have been taken up as of May 25, a day before it is opened to the rest of the adult population in those states.
If you are on a waiting list since the first round of AstraZeneca opt-in registrations, you will be automatically allocated an appointment in this phase of the vaccine rollout.
Where is this happening?
The AstraZeneca vaccine administration centres are at the following locations:
- Setia Spice Convention Centre, Penang
- Persada Johor International Convention Centre, Johor Bahru
- Borneo Convention Centre Kuching, Sarawak
- Institut Kemahiran Belia Negara Miri, Sarawak
- World Trade Centre Kuala Lumpur
- University of Malaya, Kuala Lumpur
- Universiti Kebangsaan Malaysia, Bangi, Selangor
- Ideal Convention Centre, Shah Alam, Selangor
How do I sign up?
There are several ways to sign up for the AstraZeneca vaccine once registration opens at 12pm.
One is to register at the Special Committee on Ensuring Access to Covid-19 Vaccine Supply’s (JKJAV) website, which is available in English, Malay, Chinese, and Tamil languages.
You should have your IC/passport details, date of birth, and phone number ready to facilitate the registration process.
A portion of the appointments has been set aside for registrations through the district health office's community outreach programmes, and some vaccines will be sent to elderly care homes.
Some state governments are also running outreach programmes to help people register for vaccination.
The Selangor government is recruiting volunteers under its ImuniSel initiative to assist those who don't have their own smartphones, have poor IT literacy, or have limited internet access.
This includes assisting elderly folks with registration for the AstraZeneca vaccine.
What happens when you sign up online?
After clicking "Book now" on the website, you will be shown the terms and conditions of your registration for the vaccination.
If you agree, you will then be prompted to provide your IC or passport number, date of birth, and phone number.
Then, you will be able to choose the venue and date for your vaccine appointment. Click "Submit" once everything is in order.
If your registration is successful, you will be shown an acknowledgement message.
At some point afterwards, you will be notified via MySejahtera or SMS on the time of your appointment. You will have 48 hours to confirm the appointment by replying to the SMS or via MySejahtera, or it will be allocated to someone else.

As your appointment date approaches, you should expect to receive reminders via MySejahtera, SMS, and automated phone calls.
To ensure a smooth vaccination, you should arrive 15 to 30 minutes before the appointment. You are discouraged from arriving earlier than that to avoid overcrowding the vaccine administration centre.
You should also bring along a pen to fill in some paperwork, and your IC or passport. If you are a MySejahtera user, you should bring along your smartphone too.
If you also have a history of a weakened immune system, severe allergic reactions (anaphylaxis) or bleeding disorders, you should see your regular doctor or specialist before your appointment for a pre-vaccination assessment. This is applicable regardless of what vaccine you’re getting.
The AstraZeneca vaccine is not recommended for pregnant women but can be used by breastfeeding women.
Once you arrive at the appointed time and place, a doctor will assess your health to determine whether you are suitable to receive the AstraZeneca vaccine.
If you are deemed suitable and have been given the vaccine, you should expect your second dose in 12 weeks. Please note that this interval is longer than other vaccines available under NIP (details below).
If you are deemed ineligible, you will be returned to your place in the queue for the mainstream vaccination programme.
What happens if you don’t sign up?
Assuming that you have already signed up for NIP but have yet to be vaccinated, you will remain in line to receive the Covid-19 vaccine from either Pfizer-BioNTech or the one from Sinovac.
The same applies if you have signed up for the AstraZeneca vaccine but are deemed unsuited for the vaccine.
The government is also considering vaccines from CanSino Biologics and Russia’s Sputnik V vaccine for NIP but these have yet to receive regulatory approval.

For the current phase of the mainstream vaccination programme, the government is prioritising people above 60 years old, people with chronic illnesses, people with disabilities and frontliners who missed out on the first phase of the vaccination programme.
The programme has been picking up pace with 39,781 doses administered per day over the past 14 days.
The figure includes the Hari Raya holidays where very few vaccines were administered, and both the mainstream and AstraZeneca vaccinations to date.
The pace is expected to go even faster in June when more vaccine supplies are delivered.
You can track the progress of the vaccination programme through Malaysiakini's Covid-19 tracker, here.
What are the benefits of signing up for the AstraZeneca vaccine?
It may be an attractive proposition for some people to have certainty on when, where and what vaccine they will get.
For those who don’t want to wait much longer for their turn in the mainstream vaccine queue, this alternative channel provides an avenue to get vaccinated much sooner.
This is especially the case for adults below 60 years old, if appointments are still available after May 26.
Though, as noted above, the second dose would only come 12 weeks later.
What if I get exposed to Covid-19 while waiting for my second dose? How well does this vaccine work?
The AstraZeneca vaccine’s effects are seen even from the first dose, even though the standard dosing regimen calls for two doses to be administered four weeks apart.
An analysis published in The Lancet in March found that it has an efficacy of at least 76.7 percent in preventing symptomatic disease 21 days after the first dose and the protection lasts at least 90 days after the injection.
More recently, the UK government agency Public Health England looked into Covid-19 transmission in real-world scenarios for those who received just one dose of either the Pfizer or the AstraZeneca vaccine.
It reported that if a vaccine recipient tests positive for Covid-19 three weeks or more after receiving one dose of the AstraZeneca vaccine, they are 38 percent less likely to pass on the disease to their unvaccinated household members. The Pfizer vaccine fared better with a 49 percent risk reduction.
Instances of Covid-19 deaths and hospitalisations are rare even after the first dose. Public Health England said deaths fell by 80 percent after the first dose.
With two full doses administered, various clinical trials have reported efficacy against symptomatic Covid-19 ranging from 62 to 79 percent, as well as 100 percent protection against Covid-19 hospitalisation and death.
Meanwhile, the March study mentioned earlier also found that the vaccine’s efficacy is improved when the two doses are given 12 or more weeks apart, compared to shorter intervals between doses.
It found that the efficacy is 55.1 percent when the doses were administered less than six weeks apart, but this improves with increasing intervals up to 81.3 percent when administered 12 or more weeks apart.
Meanwhile, Public Health England said two doses of the vaccine provided around 85 to 90 percent protection against symptomatic disease in the "real world". The doses are administered 12 weeks apart in the UK.
For the record, 12 weeks is the maximum dosing interval recommended by the World Health Organisation, which ranges from four to 12 weeks.
What about the risk of blood clots?
Health regulators around the world now recognise a link between the AstraZeneca vaccine and exceedingly rare but potentially life-threatening blood clots known as thrombosis with thrombocytopenia syndrome (TTS). This link is believed likely to be causal and not a mere coincidence.
However, the same regulators also endorse the continued use of the vaccine, stating its benefits in preventing Covid-19 far outweighed its risks.
The UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) said up to May 12, the incidence of such blood clots is 12.3 cases for every million first-doses of the vaccine (including cases where it is unknown whether the clots occurred after the first or second dose).
At that point, the UK has administered 23.0 million first doses of the vaccine, and 9.0 million second doses of the vaccine. Of these, 309 cases of TTS was reported, of which 56 people (18 percent) died.
The incidence is higher among young adults than in older people, and women are slightly more prone to TTS than men.
It has issued guidelines for vaccine recipients and healthcare workers to help spot this rare side effect. Doctors are told to refer such patients to a haematology specialist urgently.
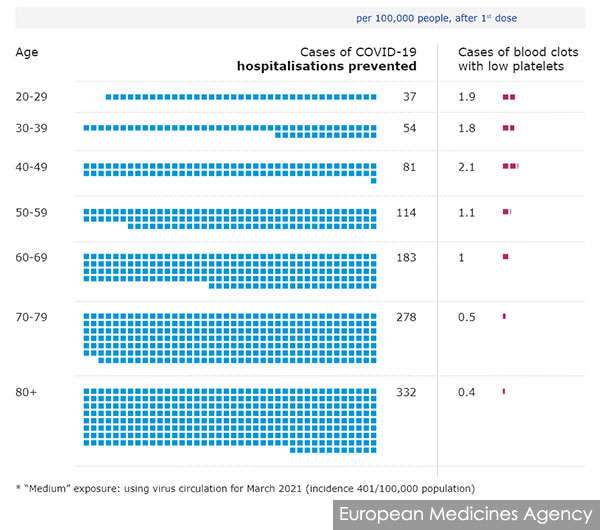
Meanwhile, the European Medicines Agency said the risk of the rare blood clots is about 1-in-100,000, varying slightly by age.
It estimated that in a medium-exposure scenario where there are only 401 Covid-19 cases per 100,000 population in a month, the vaccine would prevent 37 hospitalisations for every 100,000 people aged 20 to 29 while causing 1.9 cases of TTS.
Older people are both at greater risk of severe Covid-19 and reduced risk of TTS. For those above 80 years old, the vaccine is expected to prevent 332 Covid-19 hospitalisations while causing 0.4 cases of TTS.
The benefits are even greater when the disease is circulating more rampantly, as more Covid-19 hospitalisations are prevented. Conversely, younger folk may see diminishing returns if the incidence is low.
The vaccine’s varying benefits for different age groups and factors such as local Covid-19 incidence are the main reasons why some countries only approve the AstraZeneca vaccine for certain age groups while others like Malaysia approved it for all adults.
For context, the incidence of Covid-19 in Kuala Lumpur for the first four months of 2021 was 1,567 cases per 100,000 people. That’s an average of 392 cases per 100,000 population per month.
Likewise, the figure in Selangor is 1,475 cases per 100,000 population over four months or 369 cases per 100,000 population per month.
To compare with other vaccines, it should be noted that the Pfizer vaccine carries a risk of severe allergic reactions (anaphylaxis) of about 11 cases per million people vaccinated.
However, this risk is somewhat mitigated by having a 15 to 30-minute observation period after the injection.
This allows life-saving medication to be administered immediately if there is an allergic reaction, which usually occurs soon after the injection.
What should I look out for?
The JKJAV issued a factsheet stating the TTS risk is 9.3 per million doses administered, which is at variance with the MHRA and EMA’s estimates.
It says you should monitor signs and symptom for four to 30 days after vaccination and seek immediate medical attention if you experience the following:
1. A severe persistent headache that:
- does not improve with simple painkillers
- gets worse when lying down or accompanied by nausea and vomiting
2. Neurological symptoms such as:
- blurred vision
- difficulty with speech
- drowsiness
- seizures
3. Shortness of breath or chest pain
4. Swelling of the legs
5. Persistent abdominal pain
6. Tiny blood spots under the skin
What if I get a blood clot?
Aside from requiring immediate medical attention, you may be entitled to a claim under the RM10 million Covid-19 Vaccine Injury Fund managed by the National Disaster Management Agency.
Under the scheme, vaccine recipients who suffer serious side effects that require long-term hospital treatment are eligible to receive up to RM50,000. Those who suffer permanent disability or death are entitled to up to RM500,000.

Are there other side effects?
As with other vaccines, side effects are usually mild and should resolve themselves in a few days. Paracetamol tablets may provide some relief if you find the fever or pain troublesome.
Very common side effects include tenderness at the injection site, fatigue, headaches, joint pain and nausea, although not everyone may experience these effects.
Around 1-in-10 may suffer fever, vomiting or diarrhoea, and flu-like symptoms. About 1-in-100 may suffer dizziness, decreased appetite, enlarged lymph nodes, and rashes.
You will be given more details about potential side effects at the PPV, including when to seek medical attention.
If you suspect you are suffering a vaccine side effect, you can report this via the MySejahtera app even if you are not sure. This is aside from seeking medical attention if necessary.
This helps regulators detect vaccine side effects even if they are very rare or had been missed during clinical trials, as the MHRA and EMA had done to investigate the AstraZeneca vaccine’s blood clot risks.
Alternatively, you may submit a report via the National Pharmaceutical Regulatory Agency’s website.
What about the new Covid-19 variants?
The AstraZeneca vaccine fared poorly against B.1.351 variant. This variant was first identified in South Africa, but 66 cases have been found circulating in Malaysia.
This variant is classified as a “variant of concern” and carries the E484K mutation that is associated with reduced vaccine efficacy against some Covid-19 vaccines and an increased chance of re-infecting Covid-19 survivors. Some vaccines are more affected than others.
READ MORE: Vaccines vs variants: What we know so far?
Trials found the AstraZeneca vaccine’s efficacy dropped to as low as 10.4 percent against this variant which prompted the South African government to suspend the use of the vaccine in favour of the vaccine developed by Johnson & Johnson.
The figure refers to mild-to-moderate illness only, as several limitations of the study meant no conclusions could be reached on the vaccine's protection against severe disease and death.
Another potential issue is the P.3 variant, first detected in the Philippines but eight cases have since been found in Sarawak. This variant’s effects are still under investigation but it, too, carries the E484K mutation.
Fortunately, these variants are not yet prevalent in the country. The prevalent variants in Malaysia - such as B.1.524 in Peninsular Malaysia and B.1.466 in Sabah and Sarawak - are not known of having an impact on vaccine efficacy.
The government is enhancing quarantine measures and genomic surveillance to ensure its portfolio of vaccines will remain effective against variants in Malaysia.
AstraZeneca is also developing updated versions of its vaccine to combat new variants.
Why the special treatment for the AstraZeneca vaccine?
Originally, the government planned to use the AstraZeneca vaccine alongside other vaccines in its portfolio. To ease logistics, recipients have no choice in what vaccine they will receive apart from refusing to sign the consent form once at the PPV.
After the vaccine’s blood clot risk had been identified, the government assessed its benefits as outweighing risks and planned to give it to people aged 60 and above as part of the second phase of NIP.
However, this came just weeks after the highly publicised blood clot risks had been identified.
Public backlash came quickly, especially when the first shipment of 268,800 AstraZeneca doses arrived on April 23. This persisted despite the government’s attempts to explain its benefits and how it outweighs the risks.
The backlash prompted a rethink of how the AstraZeneca vaccine would be deployed and avoid jeopardising the rest of the vaccination programme that is already struggling to get people to sign up.
Kuala Lumpur and Selangor have been singled out to receive the first of the AstraZeneca vaccines due to the high incidence of Covid-19 in the Klang Valley. The lengthy 12-week interval will help get the first dose of the vaccine to as many people as possible in a short time.
The upcoming round of the AstraZeneca rollout would extend the vaccine to Penang, Johor, Kuching, and Miri.
How did the first round turn out?
There was criticism regarding vaccine inequality. Since the first round of appointments can only be booked online, it essentially excluded those with poor IT literacy or who have limited access to digital devices and internet connectivity.
Another criticism was that registration is open to anyone eligible for Covid-19 vaccination, rather than prioritising senior citizens who are at greater risk of severe illness and death caused by Covid-19.
Nevertheless, the opt-in strategy achieved several important objectives.
When the first shipment of AstraZeneca vaccines arrived, about 8,000 people cancelled their registration for NIP and an increasing number of people failed to turn up for their appointments.
By May 3, a day after the registration date, Khairy said 80 percent of those who cancelled have already re-registered.
And despite the controversy surrounding the vaccine and technical difficulties with the registration process, all 268,000 appointment slots were snapped up in around three hours and 15 minutes.
Registration for the second round opens at noon here.
This instalment of KiniGuide is compiled by KOH JUN LIN.

